In this article a detailed review of Cyclodextrine polymers and it’s characteristics, uses, advantages and future trend in water treatment is discussed in detail.
Cyclodextrin polymers and their use in water treatment is getting popularity recently. Due to the increasing concern over removal of emerging contaminants like PFAS, personal care products, Pharmaceuticals, hormones etc. the use of adsorbing agents other than conventional adsorbants are gaining popularily. Cyclodextrin polymers due to it’s unique features are gaining polularity among researchers and water treatment professionals. Let’s dive into deeper and have in depth knowledge particularly in water treatment sector.
What are Cyclodextrin polymers (CDPs)?
Cyclodextrin polymers are advanced materials that are made by linking multiple cyclodextrin (CD) molecules together to create a 3-dimensional network or polymer structure. Cyclodextrins are ring-shaped molecules that are derived from starch.
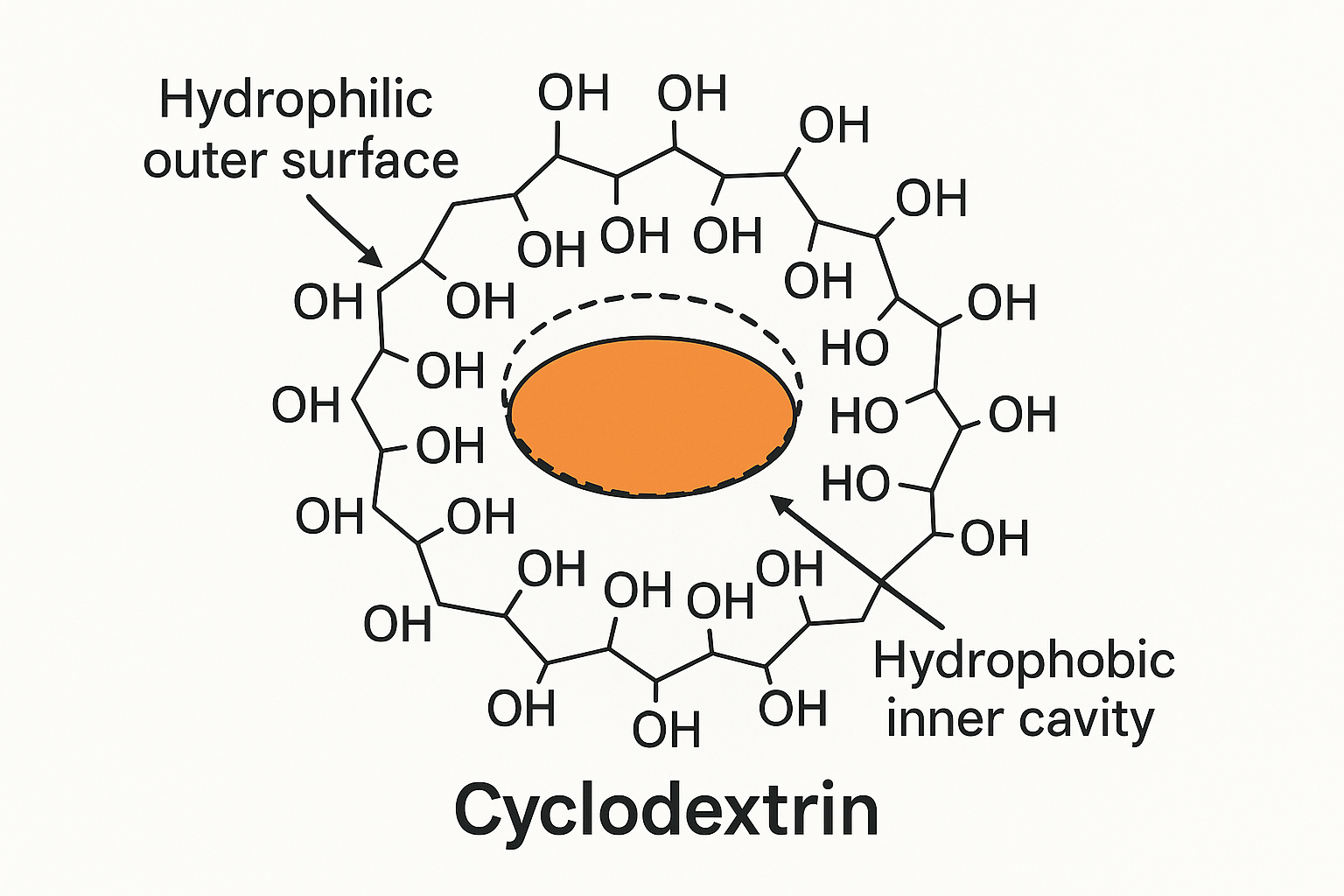
How many types of Cyclodextrine Polymers (CDPs) are there?
The most commonly used cyclodextrins are:
- α-Cyclodextrin (6 glucose units)
- β-Cyclodextrin (7 glucose units), this category is most widely used.
- γ-Cyclodextrin (8 glucose units)
Why is it called Cyclodextrin?
The term Cyclodxtrin is a combination of two parts:
- “Cyclo” – which refers to the cyclic or ring shaped structure of the molecule.
- “Dextrine” – Dextrins are short chain of glucose molecules (C₆H₁₂O₆) produced by hydrolysis of starch.
So basically Cyclodextrin term is coined because these are special type of dextrin where the glucose units are arranged in a ring
How Cyclodextrin polymers are manufactured?
Cyclodextrin polymers are manufactured by cross-linking multiple cyclodextrine molecules in large stainless steel reactors (batch or continuous flow) to form a polymer network. The steps of manufacturing these polymers are given below.
Polymerization Process
- Cyclodextrin Dispersion – to dissolve or disperse in water
- Cross-Linker Addition ( cross-linker like epichlorohydrin)
- Controlled Heating (40º C to 80º C at pH around 11-12)
- Polymer Network Formation
Post-Reaction Processing
- Washing
- Neutralisation
- Drying
- Grinding (if needed)
What types of contaminants are removed by Cyclodextrin polymers (CDPs)?
Cyclodextrin polymers are very efficient in removing trace organic pollutants from both the waste water and potable water. The list of the contaminants are given below.
- Pharmaceuticals and personal care products (PPCRs)
- Endocrine-Disrupting compounds (EDCs)
- Per- and Polyfluoroalkyl substances (PFAS)
- Pesticides and Herbicides
- Volatile organic compounds (VOCs)
- Heavy metal (Indirectly)
What are the mechanisms followed by Cyclodextrine polymers in contaminant removal?
Cyclodextrin polymers (CDPs) removes contaminants from water and other media through a number of mechanisms which are due to its unique structure and chemical properties of the polymer matrix. The key removal mechanisms are discussed below.
Host-Guest Inclusion Complexation
This is the primary mechanism for organic contaminant removal. In this mechanism the contaminant slides into the hydrophobic interior cavity of the CDPs. The interaction between the contaminant and the CDP is non-covalent and it is often reversible and the contaminants can be released by changing pH or temperature.
Hydrophobic binding
Hydrophobic contaminants come closer to the hydrophobic cavity portion of CDPs to minimise their exposure to the water. persistent organic pollutants (POP) that are too large for the cavity to slip in binds on the surface by means of hydrophobic binding and weak Van Der Waals forces.
Adsorption on polymer matrix
The cross-linked network are capable of removing contaminants by adsorption. Adsorption may occur on the functional groups on the surface or in porous structure. These are applicable for inorganic ions, dyes and bulkier compounds.
Pore entrapment
Colloidal particles and large dye molecules can be removed by physical entrapment rather than chemical interaction, this is possible because the porous CD polymers can trap larger molecules or particles that can’t enter the CD cavity.
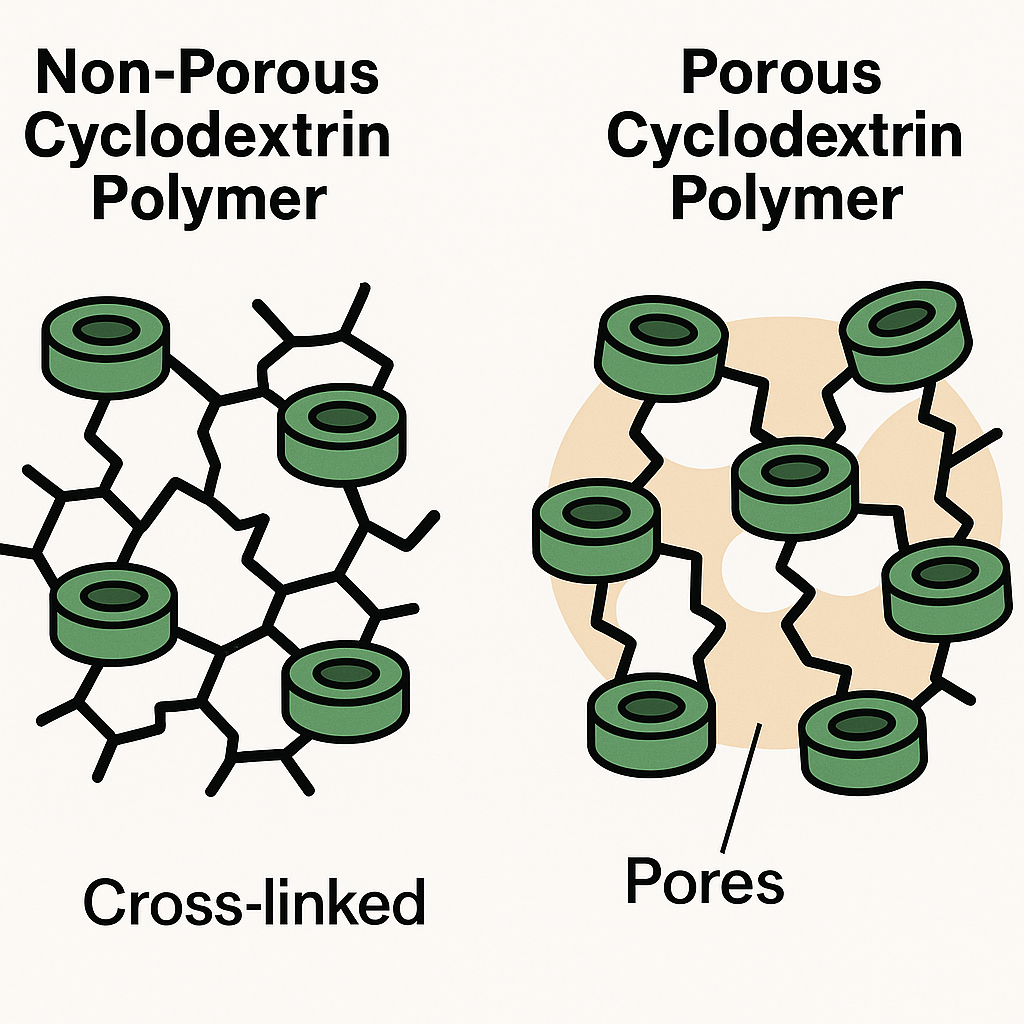
What are porous Cyclodextrin polymers (CDPs)?
Porous cyclodextrin polymers are 3D cross-linked network, built from cyclodextrin molecules that are linked by rigid aromatic or other functional cross-linkers.
What is the difference between non-porous and porous Cyclodextrin polymers (CDPs)?
Non-porous cyclodextrin polymers (CDPs) are normally made up of flexible cross linkers such as epichlorohydrin or citric acid and these create dense, compact networks which has low BET surface area (usually < 100 m²/g). These are having less porosity and lesser potential of mass transfer into cyclodextrin cavities. In these cyclodextrins the pores are mainly due to cyclodextrin cavities. These are having low adsorption potential.
Porous cyclodextrin polymers (CDPs) are normally made up of rigid or aromatic cross linkers like : phytic acid, benzyl groups etc. These are having more porous and rigid structure which increases the surface area usually ranging from 100 – 1000 m²/g. The pores are mainly due to multi – scale pores micro-meso-macro. These are having high adsorption potential.
What is the contaminant removal efficiency of Cyclodextrin polymers?
The contaminant removal efficiency of Cyclodextrin polymers (CDPs) depends on the type of contaminant, polymer structure etc. CDPs shows very high removal efficiency in removing pharmaceuticals, pesticides and endocrine disrupting compounds. A summary of contaminant removal efficiencies are given below.
| Contaminant(s) | Type of CDP | Removal Efficiency | Reference |
|---|---|---|---|
| Bisphenol A, Atrazine, Naproxen, Metolachlor, etc. | β-CD + TFN crosslinker (porous CDP) | >95% (within seconds to minutes) | Alsbaiee et al. (2016) Nature DOI:10.1038/nature16933 |
| PFAS (PFOA, PFOS), dyes, pharmaceuticals | β-CD, γ-CD with various crosslinkers | 70–99% | Li et al. (2020) Environ. Sci. Nano DOI:10.1039/C9EN01193F |
| Dyes (Methylene Blue), BPA, Heavy Metals (Pb²⁺, Cd²⁺) | β-CD-based CDPs (modified & composite) | 60–95% | Ling et al. (2021) Sep. Purif. Technol. DOI:10.1016/j.seppur.2020.117772 |
| Mixed organic micropollutants | Porous β-CDP composites | 85–99% | Tang et al. (2023) ACS ES&T Water |
| Carbamazepine, Ibuprofen | β-CD + Epichlorohydrin CDP | ~80–90% | Gao et al. (2019) Chemosphere |
Comparison between Cyclodextrin Polymers vs. Conventional Adsorbents.
Compared to normal adsorbents CDPs are more efficient in removing small, polar organic micro pollutants such as BPA, Carbamazepine, PFAS etc. due to their molecular structure. On the other hand normal adsorbents like activated carbon are more efficient for no-polar or large molecules and heavy metals. CDPs are capable to work faster and they can achieve removal efficiency >95% within a very short time, while activated carbon needs longer duration and contact times.
Advantages and disadvantages of using Cyclodextrine polymers.
Cyclodextrin polymers (CPDs) are emerging materials with unique structural and functional properties, the main advantages and disadvantages are given below.
Advantages
- High selectivity for contaminants
CDPs are highly selective due to their ability to form inclusion complexes with specific organic molecules like pesticides, pharmaceuticals, dyes.
- Enhanced adsorption capacity
Cyclodextrin polymers show enhance adsorption capacity over conventional adsorbents.
- Biodegradability and low toxicity
Cyclodextrins are synthesised from starch, so they are generally considered as eco-friendly and non-toxic.
- Regenerability
CDPs are reusable and can be regenerated by altering pH and temperature.
Disadvantages
- Limited removal of Inorganic.
CDPs are mainly effective for removing organic pollutants, they are not effective for removing heavy metals, nitrates or fluorides.
- Cost of synthesis
CDPs are biodegradable however the synthesis of cross linked CDPs can be costly and time consuming.
- Competitive adsorption
If multiple organic pollutants are present then it leads to competition among them to get attached into the cyclodextrin cavity which will reduce its overall efficiency.
Can Cyclodextrin polymers be manufactured commercially?
Cyclodextrin polymers are commercially manufactured, however their production is still limited compared to the conventional polymers. Companies like CycloPure Inc. (USA), they manufactures DEXSORB® which is a β-CD polymer used for removing PFAS, pharmaceuticals and pesticides from water.
Conclusion
Cyclodextrin polymers are very promising and eco-friendly advanced material for water treatment. They offer high selectivity, rapid adsorption kinetics and reusability. Their unique porous structure makes them effective in targeting contaminants that are hard to remove by conventional methods. However large-scale implementation is still less explored. Future research should focus on large-scale production techniques.
Read More: Contaminant Adsorption: Cutting-Edge Water Treatment and The Role of Chitosan and its derivatives